You Know What Gets on My Nerves Myelin
The myelin sheath is a greatly extended and modified plasma membrane wrapped effectually the nervus axon in a screw style [1]. The myelin membranes originate from and are a part of the Schwann cells in the peripheral nervous system (PNS) and the oligodendroglial cells in the primal nervous system (CNS) (run across Chap. 1). Each myelin-generating prison cell furnishes myelin for but one segment of any given axon. The periodic interruptions where short portions of the axon are left uncovered past myelin are the nodes of Ranvier, and they are critical to the functioning of myelin.
Myelin facilitates conduction
Myelin is an electrical insulator; however, its office of facilitating conduction in axons has no exact analogy in electrical circuitry. In unmyelinated fibers, impulse conduction is propagated by local circuits of ion electric current that menstruation into the agile region of the axonal membrane, through the axon and out through adjacent sections of the membrane (Fig. 4-1). These local circuits depolarize the adjacent piece of membrane in a continuous, sequential way. In myelinated axons, the excitable axonal membrane is exposed to the extracellular infinite only at the nodes of Ranvier; this is the location of sodium channels [ii]. When the membrane at the node is excited, the local circuit generated cannot menstruum through the high-resistance sheath and, therefore, flows out through and depolarizes the membrane at the next node, which might be 1 mm or farther abroad (Fig. four-1). The low capacitance of the sheath means that piddling energy is required to depolarize the remaining membrane between the nodes, which results in local circuit spreading at an increased speed. Active excitation of the axonal membrane jumps from node to node; this grade of impulse propagation is called saltatory conduction (Latin saltare, "to jump"). Such move of the wave of depolarization is much more rapid than in unmyelinated fibers. Furthermore, because only the nodes of Ranvier are excited during conduction in myelinated fibers, Na+ flux into the nerve is much less than in unmyelinated fibers, where the entire membrane is involved. An example of the advantage of myelination is obtained by comparison of two different nerve fibers, both of which conduct at 25 m/sec at 20°C. The 500-mm diameter unmyelinated giant axon of the squid requires v,000 times as much energy and occupies about i,500 times as much space as the 12-mm diameter myelinated nerve in the frog.
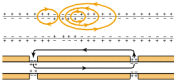
Figure iv-1
Impulse conduction in unmyelinated (top) and myelinated (bottom) fibers. Arrows show the menstruum of activeness currents in local circuits into the agile region of the membrane. In unmyelinated fibers, the circuits catamenia through the next piece of membrane, (more...)
Conduction velocity in myelinated fibers is proportional to the diameter, while in unmyelinated fibers it is proportional to the square root of the bore. Thus, differences in energy and space requirements betwixt the two types of fiber are exaggerated at college conduction velocities. If fretfulness were not myelinated and equivalent conduction velocities were maintained, the human spinal cord would need to be as big as a good-sized tree trunk. Myelin, then, facilitates conduction while conserving space and free energy [3].
Myelin has a characteristic ultrastructure
Myelin, as well as many of its morphological features, such as nodes of Ranvier and Schmidt-Lantermann clefts, can be seen readily with low-cal microscopy (Fig. 4-2). Further insight comes from biophysical studies of structures with parallel axons: sciatic nerve as representative of the PNS and optic nerve or tract equally representative of the CNS. Myelin, when examined by polarized lite, exhibits both a lipid-dependent and a protein-dependent birefringence. Low-angle X-ray diffraction studies of myelin provide electron-density plots of the repeating unit that show three peaks which stand for to protein plus lipid polar groups and ii troughs which correspond to lipid hydrocarbon chains. The repeat distance varies somewhat depending on the species and whether the sample is from the CNS or the PNS. Thus, the results from these two techniques are consistent with a poly peptide—lipid—protein—lipid—protein construction, in which the lipid portion is a bimolecular leaflet and side by side poly peptide layers are unlike in some mode. Data for mammalian optic nerve show a repeat altitude of eighty Å (Fig. 4-3). This spacing can conform one bimolecular layer of lipid (well-nigh l Å) and two protein layers (nearly fifteen Å each). The main repeating unit of two such fused unit membranes is twice this figure, or 160 Å [5]. Although it is useful to think of myelin in terms of alternating protein and lipid layers, this concept has been modified somewhat to be compatible with the "fluid mosaic" model of membrane construction, which includes intrinsic transmembrane proteins as well equally extrinsic proteins.

Figure 4-2
Light micrograph of a 1-μm Epon section of rabbit peripheral nerve (inductive root) stained with toluidine bluish. The myelin sheath appears as a thick black band effectually the stake axon. ×600, before 30% reduction. (Courtesy of Dr. Cedric Raine.) (more...)
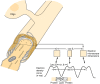
Figure 4-iii
A composite diagram summarizing some of the ultrastructural data on CNS myelin. At the top, an oligodendroglial cell is shown connected to the sheath by a procedure. The cutaway view of the myelin and axon illustrates the relationship of these two structures (more...)
Information concerning myelin structure is as well available from electron-microscopic studies, which visualize myelin every bit a series of protein layers appearing as alternating night and less night lines separated by lipid hydrocarbon chains which announced as unstained zones (Figs. 4-4–4-7). There is asymmetry in the staining of the poly peptide layers. The less dark, or intraperiod, line represents the closely apposed outer protein coats of the original cell membrane; the membranes are non actually fused since they can be resolved equally a double line at loftier resolution (Figs. 4-vi and iv-7). The night, or major period, line is the fused, inner poly peptide coat of the prison cell membrane. The echo distances observed by electron microscopy are less than those calculated from the low-angle Ten-ray diffraction data, a consequence of the considerable shrinkage that takes place subsequently fixation and dehydration. However, the difference in periodicity betwixt PNS and CNS myelin is maintained; peripheral myelin has an average repeat distance of 119 Å and central myelin, 107 Å.
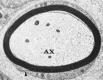
Figure 4-4
Electron micrograph of a single peripheral nerve cobweb from rabbit. Annotation that the myelin sheath has a lamellated structure and is surrounded past Schwann prison cell cytoplasm. The outer mesaxon (arrowhead) can be seen at the lower left. AX, axon. ×18,000. (more...)

Effigy 4-seven
A typical CNS myelinated cobweb from the spinal cord of an adult dog. Contrast this effigy with the PNS fiber in Effigy 4-iii. The course of the flattened oligodendrocytic process, beginning at the outer tongue (arrow), tin can be traced. Note that the cobweb (more...)

Effigy 4-vi
Magnification of the myelin sheath of Effigy 4-iv. Note that the intraperiod line (arrows) at this high resolution is a double structure. ×350,000. (Courtesy of Dr. Cedric Raine.)

Figure 4-v
College magnification of Effigy four-4 to testify the Schwann cell cytoplasm covered by basal lamina (arrows). ×50,000.
Nodes of Ranvier. 2 adjacent segments of myelin on 1 axon are separated past a node of Ranvier. In this region, the axon is not covered by myelin. At the paranodal region and the Schmidt-Lantermann clefts, the cytoplasmic surfaces of myelin are not compacted and Schwann or glial cell cytoplasm is included inside the sheath. To visualize these structures, one may refer to Figures 4-viii and 4-9, which show that if myelin were unrolled from the axon, information technology would be a flat, spade-shaped sail surrounded by a tube of cytoplasm. Thus, as shown in electron micrographs of longitudinal sections of axon paranodal regions, the major dense line formed by apposition of the cytoplasmic faces opens upwardly at the edges of the sheet, enclosing cytoplasm within a loop (encounter Figs. 4-3 and iv-9). These loop-shaped terminations of the sheath at the node are chosen lateral loops. The loops course membrane complexes with the axolemma called transverse bands, whereas myelin in the internodal region is separated from the axon by a gap of periaxonal space. The transverse bands are helical structures that seal the myelin to the axolemma only provide, by spaces between them, a tortuous path from the extracellular infinite to the periaxonal space.
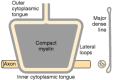
Figure 4-8
A diagram showing the appearance of CNS myelin if it were unrolled from the axon. One can visualize this structure arising from Effigy 4-3 if the glial prison cell procedure were pulled straight up and the myelin layers separated at the intermediate period line. (more than...)

Effigy 4-9
A diagram similar to Effigy 4-8 only showing one Schwann cell and its myelin sheath unrolled from a peripheral axon. The sail of PNS myelin is, similar CNS myelin, surrounded by a tube of cytoplasm and has boosted tubes of cytoplasm, which brand up the (more...)
Schmidt-Lantermann clefts are structures where the cytoplasmic surfaces of the myelin sheath take not compacted to form the major dumbo line and, instead, contain Schwann or glial cell cytoplasm (Fig. 4-9). These regions are common in peripheral myelinated axons only rare in the CNS. These inclusions of cytoplasm are present in each layer of myelin. The clefts tin can be visualized in the unrolled myelin sheet as tubes of cytoplasm similar to the tubes making up the lateral loops but in the middle regions of the canvas, rather than at the edges (Fig. four-9).
Myelin is an extension of a cell membrane
In the PNS, myelination is preceded by invasion of the nerve parcel by Schwann cells, rapid multiplication of these cells and segregation of the individual axons by Schwann cell processes. Smaller axons (≤i μm), which will remain unmyelinated, are segregated; several may be enclosed in one cell, each inside its own pocket, like to the structure shown in Figure 4-10A. Large axons (≥1 μm) destined for myelination are enclosed singly, ane cell per axon per internode. These cells line upwardly along the axons with intervals between them; the intervals become the nodes of Ranvier.
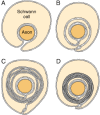
Figure 4-x
Myelin germination in the peripheral nervous arrangement. A: The Schwann prison cell has surrounded the axon, but the external surfaces of the plasma membrane take not notwithstanding fused in the mesaxon. B: The mesaxon has fused into a five-layered structure and spiraled in one case (more...)
Earlier myelination, the axon lies in an invagination of the Schwann cell (Fig. 4-10A). The plasmalemma of the jail cell then surrounds the axon and joins to form a double-membrane structure that communicates with the jail cell surface. This structure, called the mesaxon, elongates around the axon in a screw fashion (Fig. 4-ten). Thus, formation of myelin topologically resembles rolling up a sleeping purse; the mesaxon winds most the axon, and the cytoplasmic surfaces condense into a compact myelin sheath and class the major dense line. The two external surfaces grade the myelin intraperiod line.
In the CNS, the structures of myelin are formed by the oligodendroglial cell [vii]. This has many similarities only also points of divergence with respect to myelination in the PNS. CNS nerve fibers are not separated by connective tissue, nor are they surrounded past cell cytoplasm, and specific glial nuclei are not obviously associated with particular myelinated fibers. CNS myelin is a screw construction similar to PNS myelin; it has an inner mesaxon and an outer mesaxon that ends in a loop, or tongue, of glial cytoplasm (Fig. 4-3). Unlike the peripheral nerve, where the sheath is surrounded by Schwann prison cell cytoplasm, the cytoplasmic tongue in the CNS is restricted to a small portion of the sheath. This glial tongue is continuous with the plasma membrane of the oligodendroglial jail cell through slender processes. One glial cell can myelinate 40 or more than carve up axons [8].
Myelin deposition in the PNS may result in a single axon having up to 100 myelin layers; therefore, it is improbable that myelin is laid downwardly by a simple rotation of the Schwann jail cell nucleus effectually the axon. In the CNS, such a postulate is precluded by the fact that one glial cell can myelinate several axons. During myelination, in that location are increases in the length of the internode, the diameter of the axon and the number of myelin layers. Myelin, therefore, expands in all planes at once. Whatever mechanism to account for this growth must assume that the membrane arrangement is able to expand and contract and that layers slip over each other.
Myelin can be isolated in high yield and purity by conventional methods of subcellular fractionation
If CNS tissue is homogenized in media of low ionic strength, myelin peels off the axons and reforms in vesicles of the size range of nuclei and mitochondria. Because of their high lipid content, these myelin vesicles have the lowest intrinsic density of any membrane fraction of the nervous system. Procedures for isolation of myelin accept reward of both the large vesicle size and the low density [9].
In a widely used method, a homogenate of rodent nervous tissue, or dissected white matter in the example of larger animals, in isotonic sucrose (0.3 Grand) is layered direct onto 0.85 M sucrose and centrifuged at high speed. Mitochondria and synaptosomes sediment through the denser sucrose, and many of the smaller membrane fragments from other organelles remain in the 0.3 M sucrose layer. A crude myelin layer collects at the interface. The major impurities, microsomes and axoplasm trapped in the vesicles during the homogenization procedure are released by subjecting the myelin to osmotic daze in distilled water. The larger myelin particles tin can then exist separated from the smaller, membranous cloth by low-speed centrifugation or by repeating the density gradient centrifugation on continuous or discontinuous gradients, usually of sucrose. Preparations of purified myelin tin can exist subdivided further and arbitrarily into fractions of unlike densities by centrifugation on expanded continuous or discontinuous density gradients. These fractions differ somewhat in limerick.
Demonstration of purity for a myelin preparation includes electron-microscopic appearance; still, the difficulty of identifying small membrane vesicles of microsomes in a field of myelin membranes and the well-known sampling problems inherent in electron microscopy make this characterization unreliable later on a certain purity level has been reached.
Markers characteristic of myelin include certain proteins, lipids and enzymes described in the post-obit sections. Although such assays are useful, like electron microscopy they are not sensitive to small-scale amounts of impurities. If purity of a myelin preparation is an effect, it is of import to assay contamination of myelin past other subcellular fractions using markers such as succinic dehydrogenase for mitochondria; Na,K-ATPase and five′-nucleotidase for plasma membranes; NADH-cytochrome-C reductase for microsomes; DNA for nuclei; RNA for nuclei, ribosomes and microsomes; lactate dehydrogenase for cytosol; β-glucosidase for lysosomes; and acetylcholinesterase for neuronal fragments. Although all of these markers are low in purified myelin and gear up an outside limit for levels of contamination by other membranes, the bodily contamination may be less than calculated past such methods since low levels of many different enzymes announced to be intrinsic to myelin.
Peripheral nerve myelin tin can exist isolated by similar techniques, merely peculiarly vigorous homogenization conditions are required because of the large amounts of connective tissue and, sometimes, adipose tissue present in the nervus. The slightly bottom density of PNS myelin requires some aligning of gradient composition to prevent loss of myelin.
Source: https://www.ncbi.nlm.nih.gov/books/NBK27954/
0 Response to "You Know What Gets on My Nerves Myelin"
Post a Comment